What is Pharmacovigilance in the Pharmaceutical Industry?
Pharmacovigilance in the pharmaceutical sector is a critical facet focused on monitoring, evaluating, and ensuring the safety of medicinal products throughout their lifecycle. It involves systematic collection, analysis, and interpretation of adverse events and other drug-related information, aiming to enhance patient safety and contribute to the continuous improvement of drug quality and effectiveness.
How can companies benefit from Pharmacovigilance in Pharma?
Effective pharmacovigilance strategies enable pharmaceutical companies to fulfill regulatory requirements, enhance drug safety profiles, and maintain public trust. By implementing robust pharmacovigilance systems, companies can detect and address potential safety concerns promptly, ensuring the ongoing safety and efficacy of their products. This proactive approach not only safeguards patient well-being but also strengthens the company’s reputation within the pharmaceutical industry.
One innovative approach to pharmacovigilance involves leveraging advanced data analytics and artificial intelligence to analyze large datasets for early detection of potential safety signals. By employing cutting-edge technology, companies can enhance their ability to identify and assess adverse events, contributing to quicker and more informed decision-making. In an industry where patient safety is paramount, advanced pharmacovigilance capabilities provide a competitive edge. Companies incorporating these technologies demonstrate a commitment to proactive safety monitoring, instilling confidence in regulators, healthcare providers, and patients.
In parallel, establishing strong partnerships with regulatory authorities and healthcare professionals is pivotal for successful pharmacovigilance. Collaborative efforts ensure timely reporting, efficient information exchange, and a collective commitment to improving overall drug safety standards. In an ever-evolving regulatory landscape, companies with effective pharmacovigilance practices are better equipped to navigate complexities, gain regulatory approvals, and foster a culture of continuous improvement in drug safety.

Pharmacovigilance to Ensure Safety in Central Eastern Europe and Adriatic region with LENIS
LENIS’s commitment to pharmacovigilance extends to its operations in the Central and Eastern European (CEE) region and the Adriatic region, reflecting the company’s dedication to upholding the highest standards of drug safety.
Recognizing the unique regulatory landscapes of the CEE and Adriatic regions, LENIS employs tailored pharmacovigilance strategies. By collaborating closely with local regulatory authorities and healthcare professionals, LENIS ensures timely and accurate reporting of adverse events. This proactive engagement enhances LENIS’s ability to navigate diverse regulatory requirements, fostering compliance and maintaining a strong safety record.
LENIS’s pharmacovigilance initiatives also involve leveraging advanced analytics and artificial intelligence tools to analyze data comprehensively. This data-driven approach enables early detection of potential safety signals, allowing for swift intervention and risk mitigation. In a region where regulatory nuances may vary, LENIS’s commitment to cutting-edge pharmacovigilance practices positions the company as a trusted partner, dedicated to ensuring the safety and well-being of patients across the CEE and Adriatic regions.
Pharmacovigilance Coverage in EU and non-EU countries
LENIS’s pharmacovigilance reach extends across Slovenia, Croatia, Bosnia and Herzegovina, Serbia, North Macedonia, Kosovo, Montenegro, and Albania, demonstrating a comprehensive approach to drug safety management in both EU and non-EU territories.
In addition to the Adriatic region, LENIS seamlessly integrates pharmacovigilance practices into other smaller EU countries under a unified distribution agreement. The company’s experienced pharmacovigilance team collaborates with distribution partners, ensuring the consistent application of safety monitoring processes.
LENIS’s commitment to pharmacovigilance is evident in its successful implementation of safety measures in diverse regions, including the Central and Eastern European (CEE) countries such as Hungary, Czech Republic, Slovakia, Romania, Bulgaria, and Poland. Furthermore, LENIS extends its pharmacovigilance initiatives to cover the Baltic and Nordic states, reinforcing a robust safety framework across these territories.
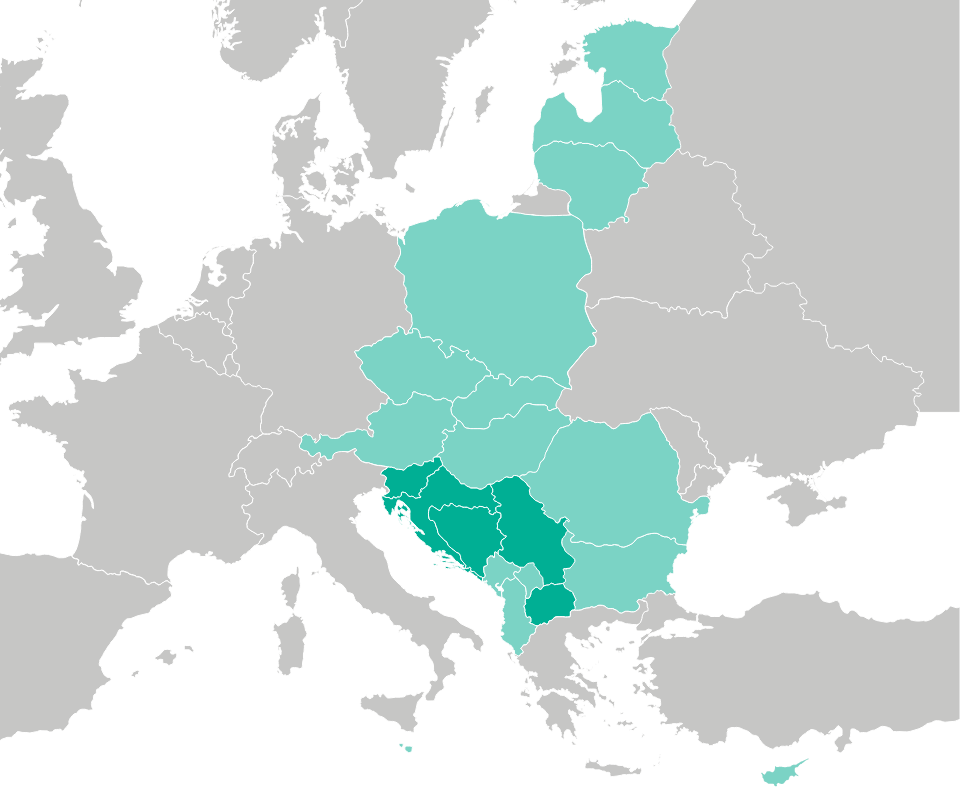
(Dark green color) Own affiliates, (Light green color) Stable partnerships
The Significance of Pharmacovigilance in Pharma Success
Pharmacovigilance plays a pivotal role in ensuring the success and sustainability of pharmaceutical companies. It not only fulfills regulatory obligations but also fosters a culture of continuous improvement, instilling confidence in stakeholders and the wider healthcare community.
Incorporating advanced technologies in pharmacovigilance enhances companies’ ability to monitor and respond to safety concerns swiftly. This proactive approach not only safeguards patient health but also positions companies as leaders in prioritizing safety within the industry.
Building strong collaborations with regulatory authorities, healthcare professionals, and other stakeholders is crucial for successful pharmacovigilance. By fostering open communication and transparent reporting, companies contribute to the collective goal of improving drug safety standards. In an era where patient trust is paramount, effective pharmacovigilance practices differentiate companies, ensuring their long-term success and positive impact on public health.
The Importance of Pharmacovigilance in Central Eastern Europe and Adriatic Regions
Pharmacovigilance holds immense importance in the dynamic markets of Central Eastern Europe (CEE) and the Adriatic (Adria) regions, where diverse healthcare systems and regulatory frameworks require tailored approaches.
The pharmaceutical industry’s increasing focus on these regions is driven by rising healthcare expenditures, growing demand for innovative treatments, and evolving regulatory landscapes. Successful market entry in CEE and Adriatic countries necessitates robust pharmacovigilance strategies that navigate regulatory complexities, ensuring compliance and rapid approvals.
LENIS’s commitment to pharmacovigilance in the CEE and Adriatic regions underscores the company’s dedication to upholding the highest safety standards. By tailoring pharmacovigilance practices to local requirements, collaborating closely with regulatory authorities, and leveraging advanced technologies, LENIS positions itself as a trusted partner in ensuring the safety and well-being of patients in these dynamic regions.
